UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of Earliest Event Reported): October 14, 2020
NURIX THERAPEUTICS, INC.
(Exact Name of Registrant as Specified in its Charter)
| Delaware | 001-39398 | 27-0838048 | ||
| (State or Other Jurisdiction of Incorporation or Organization) |
(Commission File Number) |
(IRS Employer Identification No.) |
| 1700 Owens Street, Suite 205 San Francisco, California |
94158 | |
| (Address of Principal Executive Offices) | (Zip Code) |
(415) 660-5320
(Registrant’s Telephone Number, Including Area Code)
N/A
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading symbol(s) |
Name of each exchange on which registered | ||
| Common Stock, $0.001 par value per share | NRIX | Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 | Regulation FD Disclosure. |
On October 14, 2020, Nurix Therapeutics, Inc. (the “Company”) presented at the 3rd Targeted Protein Degradation Summit (the “Presentation”). A copy of the Presentation, which will be available on the Company’s website at www.nurixtx.com under the tab “Investor,” is attached hereto as Exhibit 99.1, and is incorporated herein by reference.
The information furnished with this Item 7.01, including Exhibit 99.1, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, regardless of any general incorporation language in such filing.
| Item 9.01 | Financial Statements and Exhibits. |
| (d) | Exhibits |
| Exhibit No. |
Exhibit Title or Description | |
| 99.1 | Presentation dated October 14, 2020 | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended the Registrant has duly caused this Report to be signed on its behalf by the undersigned hereunto duly authorized.
| NURIX THERAPEUTICS, INC. | ||||||
| Date: October 14, 2020 | By: | /s/ Christine Ring | ||||
| Christine Ring, Ph.D., J.D. | ||||||
| General Counsel | ||||||

Nurix Therapeutics Blazing a New Path in Medicine Targeted Protein Modulation: Harnessing or Inhibiting E3 Ligases to Decrease or Increase Protein Levels TPD Conference 2020 Exhibit 99.1

Important Notice and Disclaimers This presentation contains forward-looking statements and information relating to Nurix Therapeutics, Inc. (the “Company,” “we," "us" or "our"). Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, our development plans, our preclinical results and other future conditions. All statements, other than statements of historical facts, contained in this presentation are forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including statements regarding our future financial or business performance, conditions, plans, prospects, trends or strategies and other financial and business matters; our current and prospective product candidates; the timing of our planned IND submissions for our product candidates; the potential advantages of our DELigase™ platform and product candidates, including NX-1607 and NX-0255; the extent animal model data predicts human efficacy; and the success and timing of our development and commercialization of our product candidates, including our DeTIL and DeCART opportunities. In addition, when or if used in this presentation, the words “may,” “could,” “should,” “anticipate,” “believe,” “estimate,” “expect,” “intend,” “plan,” “predict” and similar expressions and their variants, as they relate to the Company may identify forward-looking statements. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. Readers are cautioned that actual results, levels of activity, performance or events and circumstances could differ materially from those expressed or implied in our forward-looking statements due to a variety of factors, including risks and uncertainties related to our ability to advance our product candidates; obtain regulatory approval of and ultimately commercialize our product candidates; the timing and results of preclinical and clinical trials; our ability to fund development activities and achieve development goals; the impact of the COVID-19 pandemic on our business; our ability to protect intellectual property; and other risks and uncertainties described under the heading “Risk Factors” in our final prospectus pursuant to Rule 424(b)(4) filed with the Securities and Exchange Commission (the “SEC”) on July 24, 2020 and in our Quarterly Report on Form 10-Q for the quarter ended August 31, 2020, as well as other SEC filings. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while we believe our own internal estimates and research is reliable, such estimates and research have not been verified by any independent source. ©Nurix Therapeutics. All rights reserved.

©Nurix Therapeutics. All rights reserved. Targeting E3 ligases to develop small molecule targeted protein modulation drug candidates that can increase or decrease protein levels Four wholly-owned oncology and immunology drug candidates with first clinical trial expected to commence in H1 2021 Applying targeted protein modulation to create new adoptive cell therapies for cancer and to discover anti viral drugs DELigaseTM: a versatile drug discovery platform comprised of massive DNA-encoded libraries to screen an expanded universe of E3 ligases Revenue generating drug discovery partnerships with Sanofi and Gilead BB2 BB3 BB1 Pipeline Partners: Gilead Sanofi Technology E3 Ligase Nurix: A Targeted Protein Modulation Company *Expected IND submission timing based on calendar year quarters

DELigaseTM: Platform Enables Two Complementary Protein Modulation Approaches for Drug Discovery ©Nurix Therapeutics. All rights reserved. Protein Modulation Platform Drug Development BB2 BB3 BB1 Proprietary DNA-Encoded Libraries, >109 compounds Expanded universe of E3 ligases, >30 ligases currently in discovery Ability to push protein levels in either direction 1 2 3 Inhibitors of E3 Ligases Chimeric Targeting Molecules (CTMs) Decrease specific protein levels Ligase Inhibitors Increase specific protein levels Harnesses of E3 Ligases

DELigaseTM Identifies a Spectrum of Binders Across the Ligase Surface ©Nurix Therapeutics. All rights reserved.

Ligase Inhibitors Can Increase Levels of Specific Substrate Proteins ©Nurix Therapeutics. All rights reserved. Substrate Protein E3 Ligase Ubiquitin Natural E3 Ligase-Mediated Proteasomal Degradation Catalytic ubiquitination E3 Ligase Ligase Inhibitors Substrate Protein Substrate Protein Proteasomal Degradation Catalytic ubiquitination Nurix Ligase Inhibitor Substrate protein levels increase E3 Ligase-mediated proteasomal degradation can be highly specific to decrease substrate proteins Substrate Proteins Substrate Proteins E3 Ligase Ubiquitin E3 Ligase

Drug Candidate Target / Delivery Therapeutic Area Lead Optimization Preclinical Phase 1 Phase 2 Phase 3 Protein Degradation Chimeric Targeting Molecule (CTM) Portfolio NX-2127 BTK + IMiD activity Oral B-cell Malignancies NX-5948 BTK Oral B-cell Malignancies And GVHD Ligase Inhibition Portfolio NX-1607 CBL-B Oral Immuno-oncology DeTIL-0255 CBL-B ex vivo Adoptive Cell Therapy (ACT) ©Nurix Therapeutics. All rights reserved. *Late Q4 ‘20 – Q1 ‘21 *H2 ‘21 *H2 ‘21 *Q3 ‘21 Nurix’s Wholly-Owned Targeted Protein Modulation Drug Pipeline: Multiple Clinical Programs Expected Next Year *Expected IND submission timing based on calendar year quarters

CBL-B: A Modulator of T Cell Activation for Tumor Immunotherapy CBL-B is an E3 ligase that regulates the immune system by specifically degrading proteins involved in shutting off T-cell signaling Blocking CBL-B removes a brake on the immune system CBL-B function is supported by mouse and human genetics CBL-B inhibitors have remarkable effects on T cells CBL-B inhibitors induce immune cells to secrete IL-2 Skewing T cells to a central memory phenotype Ex vivo and in vivo administration of CBL-B inhibitors demonstrate anti-tumor effects in animal models of cancer ©Nurix Therapeutics. All rights reserved. CD80/86 CBL-B Activation MHC TCR CD28 CBL-B Signal 2 Signal 1 APC T cell Small molecule inhibitor
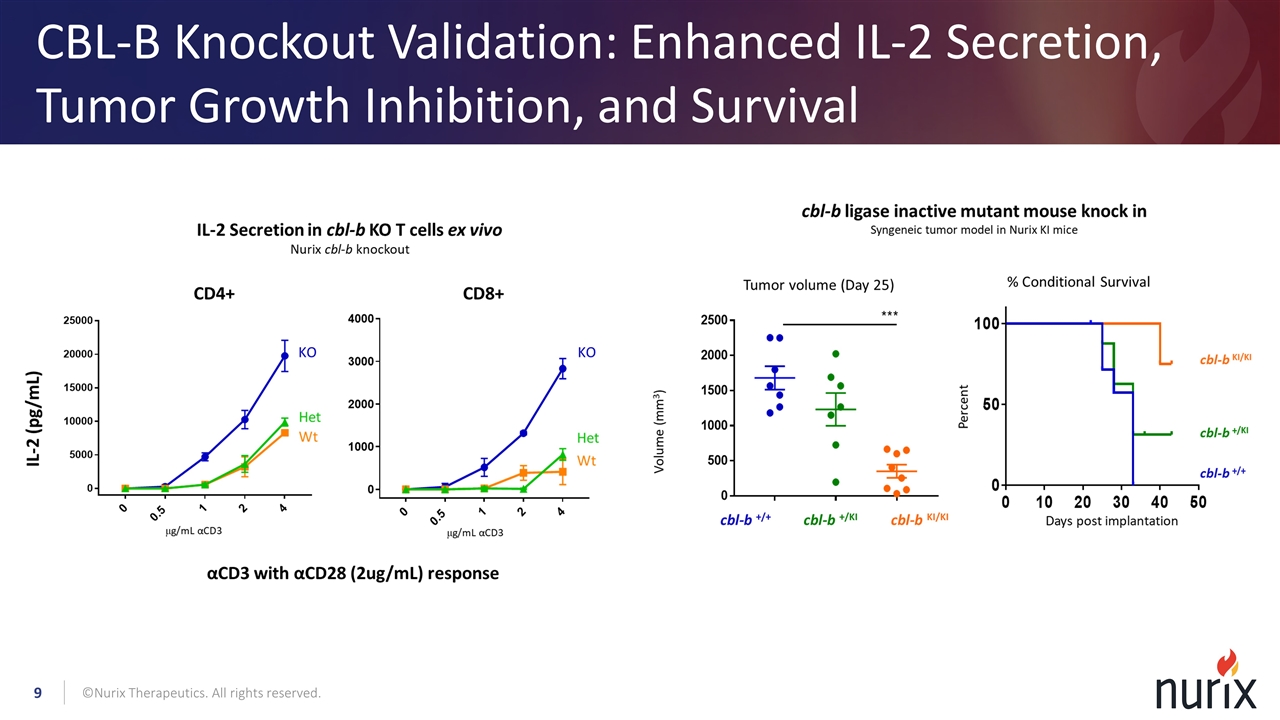
CBL-B Knockout Validation: Enhanced IL-2 Secretion, Tumor Growth Inhibition, and Survival ©Nurix Therapeutics. All rights reserved.

Nurix CBL-B Inhibitors Elevate IL-2 Levels ex vivo in Human Donor T Cells ©Nurix Therapeutics. All rights reserved. Several fold increase in IL-2 production corresponds with increasing biochemical activity of CBL-B inhibitors CBL inhibition results in increased T cell activation in the absence of co-stimulation with CD3 and CD28, a potential advantage in a suppressive tumor microenvironment

Once Daily Oral Dosing of NX-1607 Recapitulates Anti-Tumor Effects of CBL-B, Ligase Inactive, Knock-in Mouse Model ©Nurix Therapeutics. All rights reserved. CBL-B ligase-dead, knock-in (KI) model Oral daily dosing of NX-1607 Two Way ANOVA of treatment group vs vehicle control Average tumor volumes from both flanks are depicted Tumor growth inhibition (TGI) with NX-1607 treatment; tumors implanted at Day 0; once daily oral dosing of NX-1607 was given from Day 9 to Day 28 Anti-tumor effects in ligase-inactive, knock-in (KI) mutation model

NX-1607 and Anti-PD-1 Synergize to Improve Anti-Tumor Effects and Survival of Tumor-bearing Mice ©Nurix Therapeutics. All rights reserved. **** ** * Log-rank (Mantel-Cox) test of vehicle vs treatment Combination of NX-1607 and anti-PD-1 treatment significantly improves anti-tumor response and survival in mice bearing two tumors relative to vehicle or anti-PD-1 alone Tumors from both flanks plotted Two-way ANOVA of treatment group vs vehicle control ** **** Treatment Period Anti-PD-1 antibody, Oral NX-1607 CBL-B inhibitor, and Combined Treatment

NX-1607 Induces Long Term Survival in Metastatic, Triple Negative, Breast Cancer Model ©Nurix Therapeutics. All rights reserved. Triple negative breast carcinoma cells metastasize from subcutaneous space to distant sites Survival in Metastatic Breast Cancer Model Day 15: Surgical removal of primary Log-rank (Mantel-Cox) test of treatment vs Vehicle Dosing period Once daily oral dosing of NX-1607 Tumors implanted at Day 0 Surgical removal of primary tumor at Day 15 NX-1607 was given before the surgery from day 7 to day 15 (neo-adjuvant phase) and continued after surgery until day 46.

Introducing Pharmacologic Control of Adoptive Cell Therapy with Targeted Protein Modulation ©Nurix Therapeutics. All rights reserved. Adoptive Cell Therapy Nurix Adoptive Cell Therapy Program Drug-Enhanced Tumor Infiltrating Lymphocytes Drug-Enhanced Chimeric Antigen Receptor T Cell Therapy Small molecule targeted protein modulation: CBL-B Inhibitors Convergence

NX-0255 ex vivo Treatment Provides Robust Anti-Tumor Activity in Mouse Model ©Nurix Therapeutics. All rights reserved. Improvement in Conditional Survival in Mouse ACT Tumor Model Reduction in Tumor Growth in Mouse ACT Tumor Model CD8+ cells exposed to NX-0255 alone ex vivo resulted in superior conditional survival compared to using IL-2 alone CD8+ cells exposed to NX-0255 and IL-2 combined ex vivo exert a deeper anti-tumor response NX-0255 ex vivo exposure period is only three days, anti-tumor effects persist for over a month after engraftment Animals that rejected tumor were rechallenged 80 days post ACT. All animals rejected tumor, demonstrating immunological memory

Oral NX-1607 Augments Anti-Tumor Activity Observed with ex vivo NX-0255 Combination in ACT Mouse Model ©Nurix Therapeutics. All rights reserved. Reduction in Tumor Growth in Mouse ACT Tumor Model Improvement in Conditional Survival in Mouse ACT Tumor Model Oral NX-1607 treatment once daily further enhances conditional survival and anti-tumor activity of T cells expanded for three days with recombinant IL-2 plus NX-0255 ex vivo in adoptive cell therapy mouse model

CBL-B Inhibitors to Enhance Adoptive Cell Therapy: DeTILTM and DeCARTTM ©Nurix Therapeutics. All rights reserved. Oral CBL-B inhibition For co-administration to enhance engraftment to improve anti-tumor activity or to treat relapse Ex-vivo CBL-B inhibition For enhanced isolation of T cells for TIL or CAR-T therapy Ex-vivo CBL-B inhibition For ACT expansion phase to enhance cellular phenotype General schema for growing patient T cells ex vivo for adoptive cell therapy (ACT ) Oral NX-1607 Ex vivo NX-0255 DeTIL and DeCART created by ex vivo CBL inhibition with small molecule NX-0255 producing a TIL and CAR-T cell therapy products with enhanced characteristics An oral small molecule immunotherapy drug candidate in development as a single agent or in combination with other oncology therapies including adoptive cell therapy

Matching the Right Business Strategy with Each NxACT Opportunity ©Nurix Therapeutics. All rights reserved. Drug-Enhanced Tumor Infiltrating Lymphocytes Drug-Enhanced Chimeric Antigen Receptor T Cell Therapy TIL research and development being built out in Pittsburgh and Philadelphia by industry leading cell therapy experts Key recruits bring significant cell therapy experience Michael T. Lotze, M.D. Chief Cellular Therapy Officer Formerly CSO at Iovance Robert J. Brown, M.D. Vice President of Clinical Development Formerly Allogene and Iovance Wholly owned subsidiary seeded with $3M and a license to three Nurix compounds for combination use with CAR-T to enable independent investment Industry leader and DeCART founder Dr. Carl June to lead Scientific Advisory Board Chief Operating Officer, Dana Hammill, former director of strategy and business development at the Center for Cellular Immunotherapies University of Pennsylvania where she co-managed Penn-Novartis alliance for commercialization of CART19

Summary DELigase has produced highly potent hits to ligases Specific ligase inhibitors can result in pathway-specific changes in ubiquitination status associated with potent biologic effects CBL-B inhibitors activate T cells and demonstrate single agent and combination anti-tumor activity in syngeneic models CBL-B inhibitors enable drug-enhanced adoptive cell therapy with both DeTIL and DeCART ©Nurix Therapeutics. All rights reserved. BB2 BB3 BB1 Pipeline Partners: Gilead Sanofi Technology E3 Ligase *Expected IND submission timing based on calendar year quarters

Thank you Nurix Therapeutics
